Risperdal Lawsuit
 Risperdal and Risperdal Consta, (Generic Named Risperidone) are prescription medications that are categorized as anti-psychotics. Risperdal is available in pill form, while Risperdal Consta is an injectable medication for patients who need longer term effects from the medication. Risperdal was first approved by the Food and Drug Administration (FDA) in 1993 for the treatment of schizophrenia in adults. Risperdal was later approved for treatment of Bipolar ! disorder in children and adults, Irritable dementia in the elderly, and autism spectrum disorders in children and adolescents. Risperdal has also been marketed as an off-label treatment option for people with Attention Deficit Disorder (ADD).
Risperdal and Risperdal Consta, (Generic Named Risperidone) are prescription medications that are categorized as anti-psychotics. Risperdal is available in pill form, while Risperdal Consta is an injectable medication for patients who need longer term effects from the medication. Risperdal was first approved by the Food and Drug Administration (FDA) in 1993 for the treatment of schizophrenia in adults. Risperdal was later approved for treatment of Bipolar ! disorder in children and adults, Irritable dementia in the elderly, and autism spectrum disorders in children and adolescents. Risperdal has also been marketed as an off-label treatment option for people with Attention Deficit Disorder (ADD).
Since its release, Risperdal has been associated with many serious side effects, including breast growth in boys and men, movement disorders, diabetes, and increased cardio-vascular risks. In 2006, the FDA required the makers of Risperdal to place a black box warning on the medication because of its association with serious side effects.
At this time, there are over 420 lawsuits that have been filed against the manufacturers of Risperdal for the serious side effects that the patients suffered. In addition, there have also been several serious criminal investigations and settlements regarding this medication conducted by various government entities.
Parties Involved In The Lawsuit
 Risperdal and Risperdal Consta were developed and manufactured by Janssen Pharmaceuticals. Janssen is a subsidiary of the Johnson and Johnson Company. Risperidone, the generic version of this anti-psychotic medication is manufacture by Patriot Pharmaceuticals, another subsidiary of Johnson and Johnson.
Risperdal and Risperdal Consta were developed and manufactured by Janssen Pharmaceuticals. Janssen is a subsidiary of the Johnson and Johnson Company. Risperidone, the generic version of this anti-psychotic medication is manufacture by Patriot Pharmaceuticals, another subsidiary of Johnson and Johnson.
Most lawsuits that involve breast growth (Gynecomastia) name Risperdal or Risperdal Consta as the main medications that caused harm. However, several new lawsuits have recently emerged that have also named the medication Invega, another anti-psychotic medication produced by Janssen Pharmaceuticals.
Basis For The Lawsuits
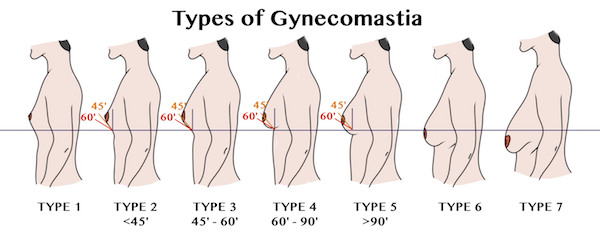
At this time, there are over 420 lawsuits filed against the makers of Risperdal. A majority of these lawsuits are related to Gynecomastia. Gynecomastia cases abnormal breast growth in young boys. Many of these boys and young men have had to undergo surgical procedures (mastectomy) to correct the problem. This has caused physical and emotional scarring to the injured parties.
Additional lawsuits filed against the manufacturer include claims that the medication caused the onset of a movement disorder known as tardive dyskinesia, high blood sugar and the onset of Type II Diabetes, Heart Attack, Stroke and Death.
The first case filed against Risperdal regarding breast enlargement came in 2008 when six young boys filed a lawsuit against the manufacturer. Of these six boys, two had undergone mastectomies to correct the problem. The other four were subjected to liposuction procedures and other forms of treatment.
In 2010, a 21 year old man filed a awsuit against Janssen Pharmaceuticals for abnormal breast growth. This man had taken this medication between the ages of eight and 14 and now suffered with enlarged breasts. His case states a very significant act: he was prescribed Risperdal as an off-label treatment and that it was not approved for the use in children at that time.
Many additional lawsuits have since been filed. Each of these lawsuits allege:
• That Risperdal has very serious side effects, including the onset of Gynecomastia, or the enlargement of breasts in young boys and men
• That Janssen Pharmaceuticals, through Johnson and Johnson, actively promoted this medication for off label uses before they were approved by the FDA.
In addition to these private lawsuits, several government lawsuits have been filed against Johnson and Johnson and Janssen Pharmaceuticals in regard to Risperdal.
In 2010, the Department of Justice and several State Attorneys filed a lawsuit against Johnson and Johnson for paying money to Omnicare for bogus services in return for Omnicare promoting the use of Risperdal in nursing homes and convalescent centers. At this time, Risperdal was not approved for the use of treating dementia patients. Johnson and Johnson settled the lawsuit with the government with a $112 million fine.
In 2011, Massachusetts and several other states filed a lawsuit against Johnson and Johnson for promoting Risperdal for off label uses and not disclosing the serious side effects, such as onset of diabetes and cardiovascular risks.
In 2012, Johnson and Johnson settled with the State of Texas for off label promoting of their medication through the Medicaid system and paid a fine of $158 million.
Also in 2012, Johnson and Johnson entered a guilty plea for a misdemeanor charge in front of the Securities and Exchange Commission. While all of this information has not yet been released, filings for the case indicate that the U.S. government invested the company for its sales and marketing practices.

 Risperdal and Risperdal Consta, (Generic Named Risperidone) are prescription medications that are categorized as anti-psychotics. Risperdal is available in pill form, while Risperdal Consta is an injectable medication for patients who need longer term effects from the medication. Risperdal was first approved by the Food and Drug Administration (FDA) in 1993 for the treatment of schizophrenia in adults. Risperdal was later approved for treatment of Bipolar ! disorder in children and adults, Irritable dementia in the elderly, and autism spectrum disorders in children and adolescents. Risperdal has also been marketed as an off-label treatment option for people with Attention Deficit Disorder (ADD).
Risperdal and Risperdal Consta, (Generic Named Risperidone) are prescription medications that are categorized as anti-psychotics. Risperdal is available in pill form, while Risperdal Consta is an injectable medication for patients who need longer term effects from the medication. Risperdal was first approved by the Food and Drug Administration (FDA) in 1993 for the treatment of schizophrenia in adults. Risperdal was later approved for treatment of Bipolar ! disorder in children and adults, Irritable dementia in the elderly, and autism spectrum disorders in children and adolescents. Risperdal has also been marketed as an off-label treatment option for people with Attention Deficit Disorder (ADD). Risperdal and Risperdal Consta were developed and manufactured by Janssen Pharmaceuticals. Janssen is a subsidiary of the Johnson and Johnson Company. Risperidone, the generic version of this anti-psychotic medication is manufacture by Patriot Pharmaceuticals, another subsidiary of Johnson and Johnson.
Risperdal and Risperdal Consta were developed and manufactured by Janssen Pharmaceuticals. Janssen is a subsidiary of the Johnson and Johnson Company. Risperidone, the generic version of this anti-psychotic medication is manufacture by Patriot Pharmaceuticals, another subsidiary of Johnson and Johnson.